Abstract
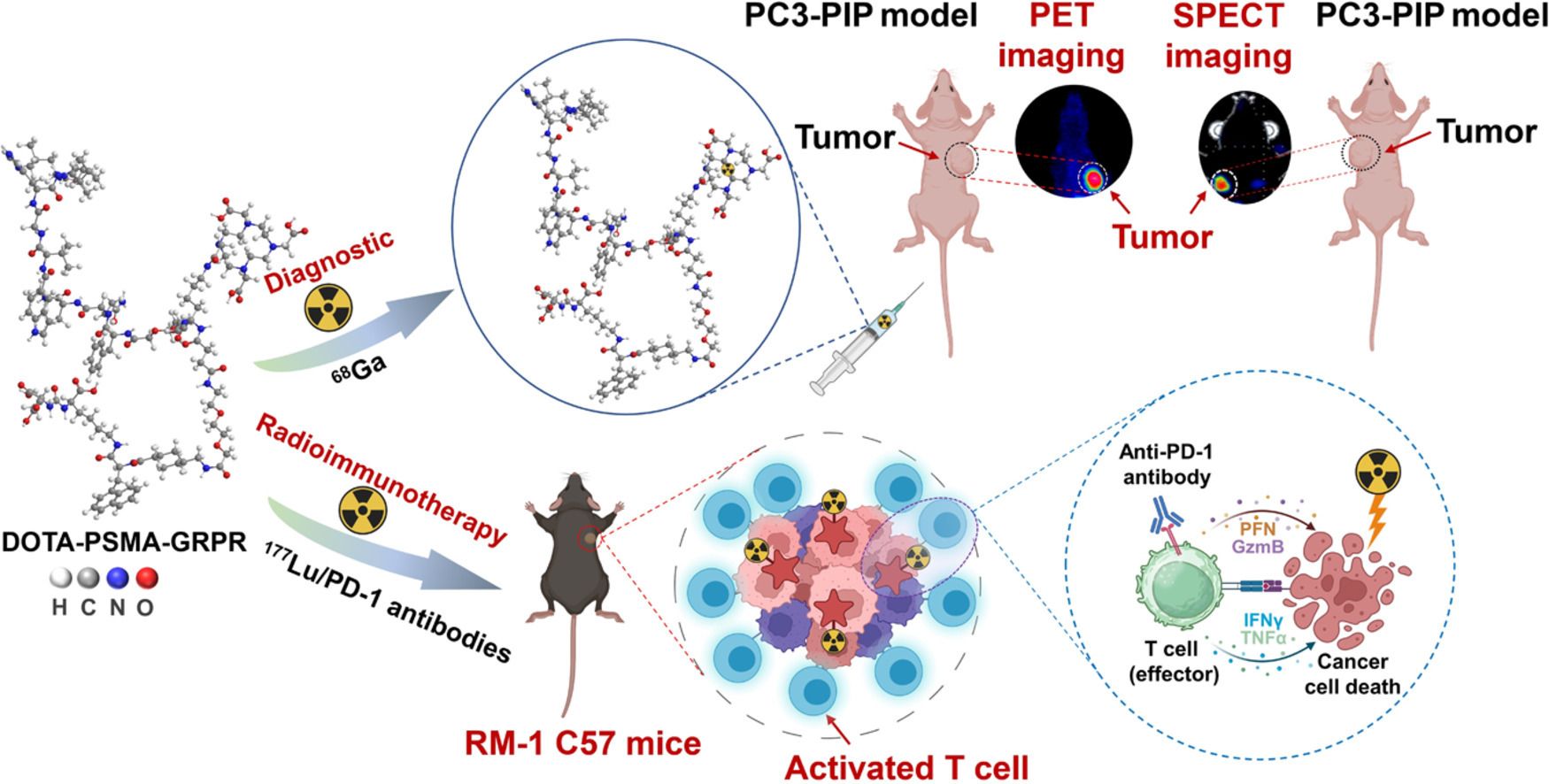
Prostate cancer poses a severe threat to the health of middle-aged and elderly males. In recent years, the development and clinical utilization of prostate-specific membrane antigen (PSMA)-targeted agents have significantly expanded diagnostic options for this malignancy. However, tumor heterogeneity substantially compromises the efficacy of radionuclide therapy, necessitating the exploration of multi-target strategies as potential solutions. This study presents a novel heterodimeric construct engineered for prostate cancer management, labeled with both diagnostic and therapeutic radionuclides to enable concurrent application in positron emission computed tomography (PET) imaging and radionuclide therapy. Our investigation primarily evaluates the therapeutic efficacy of ultra-low-dose (3.7 MBq) radionuclide therapy combined with anti-programmed cell death protein-1 (PD-1) antibody immunotherapy. In the RM-1 prostate cancer model with elevated PSMA expression, we have validated that the combination of ultra-low-dose radionuclide treatment and anti-PD-1 antibody immunotherapy yields superior therapeutic outcomes. Notably, compared to the immunotherapy-only group, the combination regimen elicited a significant increase in dendritic cells (DCs) and natural killer (NK) cells populations, with enhancements ranging from 32% to 103%. This ultra-low-dose radioimmunotherapy strategy offers a valuable theoretical framework for clinical translation, holding promise for further enhancing therapeutic efficacy in prostate cancer treatment.
文章链接:https://doi.org/10.1016/j.cclet.2025.111799